Background

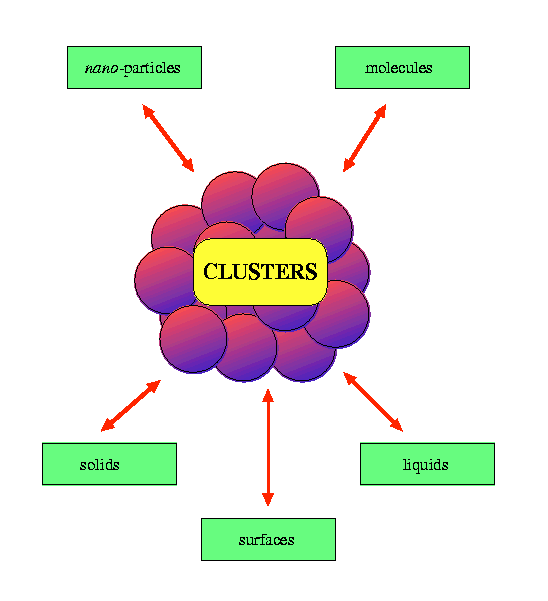
There is considerable interest in the study of elemental clusters ranging from: inert gas clusters which are held together by weak van der Waals interactions (e.g. [Ar]n); to semiconductor clusters with strong directional covalent bonds (e.g. [Si]n); and metallic clusters with fairly strong, delocalised non- directional bonding (e.g. [Na]n). Clusters are of interest for a number of reasons. Firstly, they lie between the quantum regime of small molecules with discrete energy levels and the classical regime of condensed matter where states are continuous. Clusters therefore constitute a new type of material (nano-particles) which may have properties which are distinct from those of discrete molecules or bulk matter. The study of the evolution of the geometric and electronic structures of clusters and their chemical and physical properties is also of great interest and can aid our understanding of a whole range of topics in condensed matter science. A related fundamental question is ... "how big does a cluster have to be before it behaves like the bulk element?" Finally, the high ratio of surface to interior atoms in clusters means that there are many points in common between the chemistry and physics of clusters and the surfaces of solids and liquids. The study of the adsorption and reaction of small molecules on the surfaces of transition metal clusters continues to attract much attention because of its relevance to the field of heterogeneous catalysis.
Theoretical Studies of Clusters: Theory plays an important role in cluster science. Many of the properties (such as their structures and cohesive energies) cannot be obtained directly by gas phase or molecular beam experiments, though such information may be extracted using theoretical calculations in combination with, for example, spectroscopic measurements. Clusters also provide a critical testing ground for theories and a challenge exists to formulate theoretical models of cluster bonding which cover very large size ranges (from 10-10^5 atoms). My research involves the development and application of such theoretical models in an attempt to understand how the electronic and geometric structures of semi-conductor and metallic clusters, and their physical and chemical properties, vary as a function of cluster nuclearity . Recent work in this area includes the use of empirical atomistic potentials to simulate the structures and growth patterns of carbon, silicon, aluminium and iron clusters and the modelling of the magnetism of iron clusters.